User Guide
Sign in :
Sign in by using the same user name & password in GHAD system
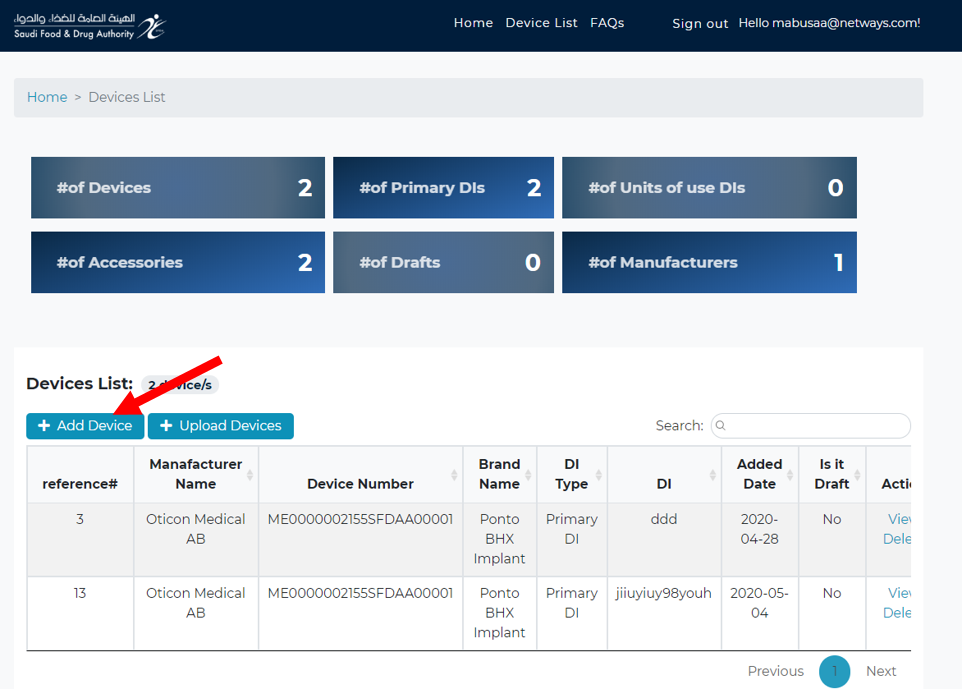
Add new device :
You can start (Add new product data) or (view ) your registered DIs data
Write a valid device listing number from GHAD database
Old MDMA listing number can’t be used
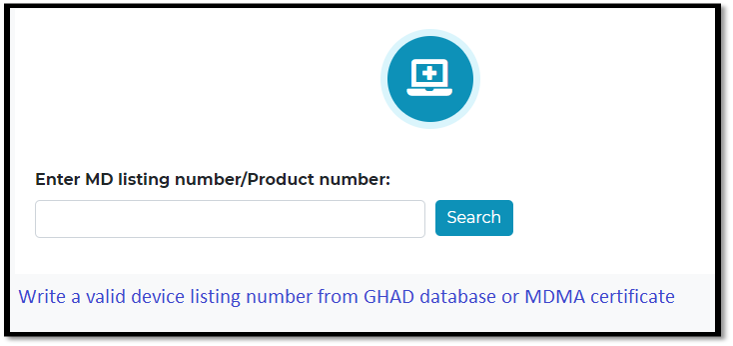
Some information will be retrieved from marketing authorization database
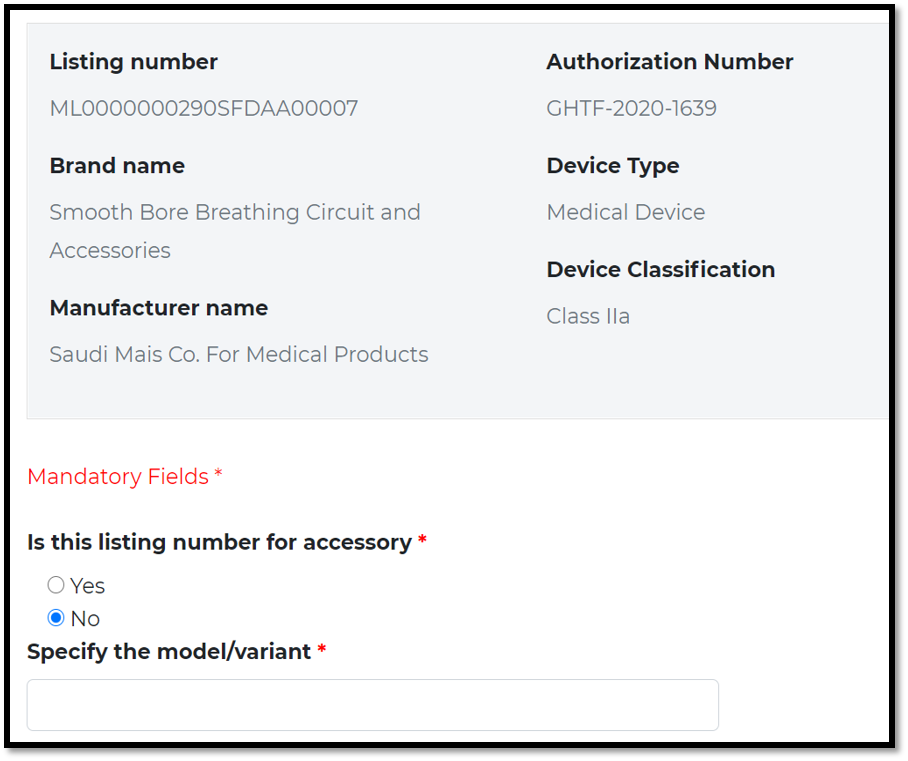
Check the shown information is correct:
Brand name / manufacturer / ….
The Listing number may include “single device” or “multiple devices ” and “accessories”. Therefore each item shall be recorded individually in Saudi-DI system. Each time you enter specific model or variant by using the same listing number
The Listing number may include “single device” or “multiple devices ” and “accessories”. Therefore each item shall be recorded individually in Saudi-DI system. Each time you enter specific model or variant by using the same listing number
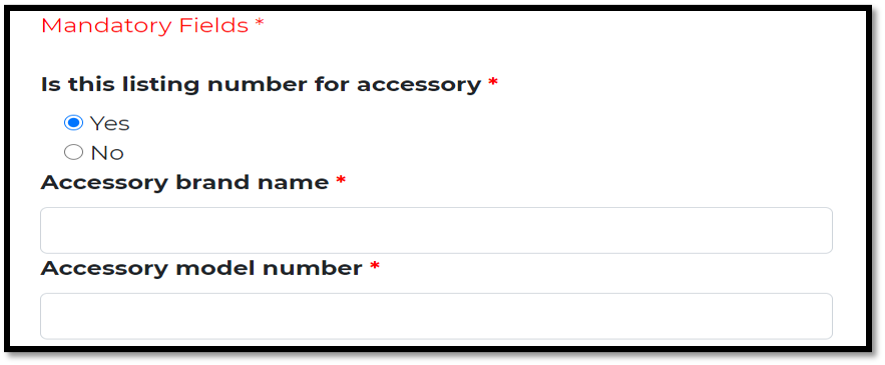
As submitted in GHAD system
If its accessory, provide name and model/ reference number.
Otherwise, provide reference number for main device /model
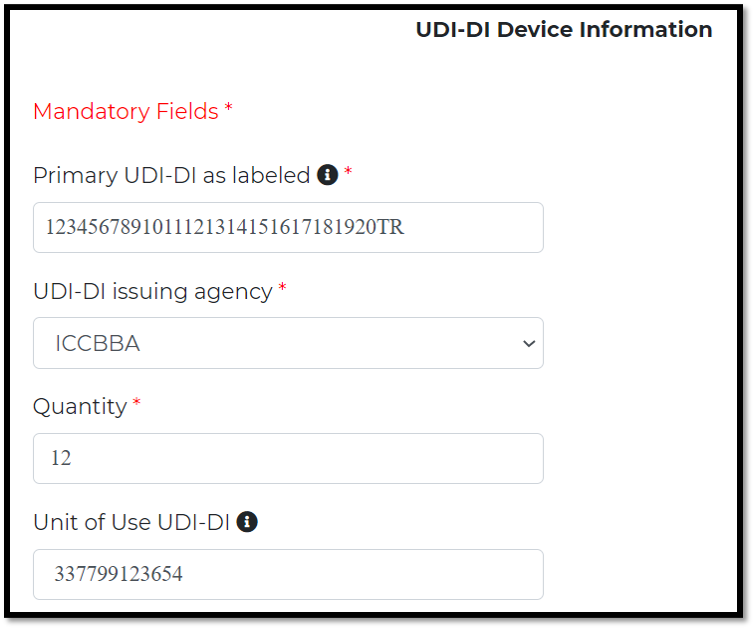
Primary UDI-DI on the device’s primary label, which consider a primary key in the database and other DIs are linked to it
Quantity: number of units in this device or package
“A Unit of Use UDI-DI” could be chosen for single-use devices if all the following conditions are met
Quantity: number of units in this device or package
- one or more.
- when more than 1 item inside the primary package, then shall provide “Unit of use UDI-DI”. Example of “ Unit of use”: a single package contains 10 pieces of similar unpacked syringes
“A Unit of Use UDI-DI” could be chosen for single-use devices if all the following conditions are met
- All are the same version or model,
- Distributed together in a single package,
- Stored in that package until removed for use,
- Not intended for individual distribution,
- Not implantable devices
As labeled, Identify one or more PIs
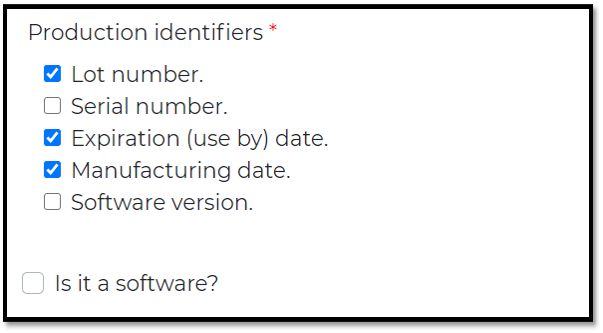
Dose the DI belong to a single item or kit or configurable device?
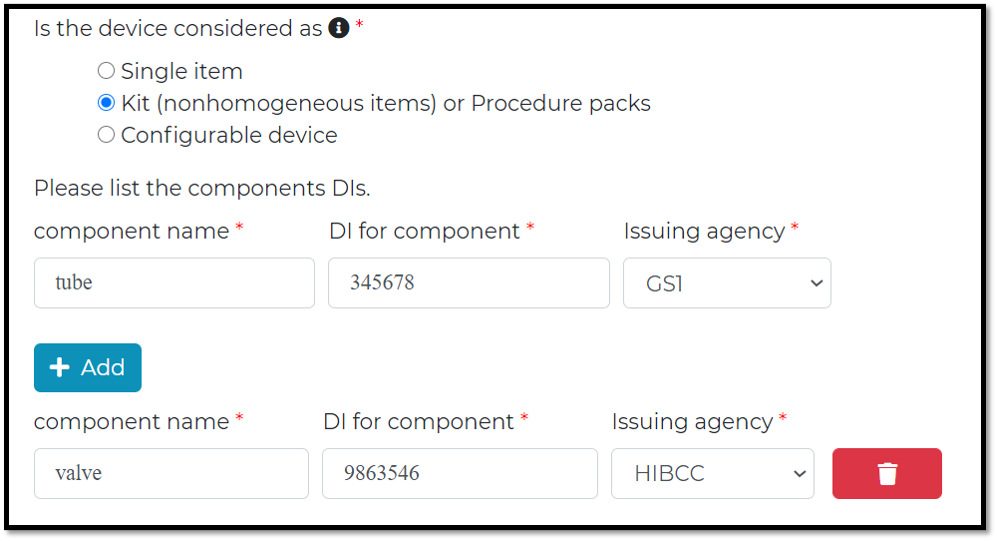
Kits and Procedure Pack (non-homogenous items in package configurations)
The UDI-DIs of all devices within the kits/packs, whether marked or not, shall be entered into the UDI database. unless the device is:
Each component, sub-system or accessory that can be removed or separated from the configuration or is available and distributed on its own (placed on the market) shall have its own, separate UDI and meet all of the other UDI requirements
The UDI-DIs of all devices within the kits/packs, whether marked or not, shall be entered into the UDI database. unless the device is:
- An individual single-use disposable device, which cannot be used outside the context of the kit or procedure pack, or
- Otherwise exempt from having a UDI on the label or package of the device that is in the kit or procedure pack.
Each component, sub-system or accessory that can be removed or separated from the configuration or is available and distributed on its own (placed on the market) shall have its own, separate UDI and meet all of the other UDI requirements
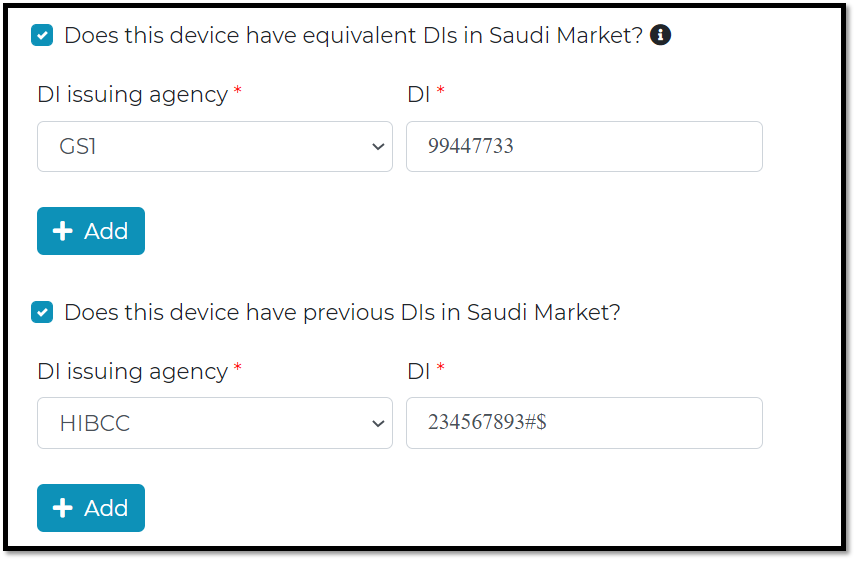
Equivalent DIs :
If the same device has been imported to KSA market with different DIs, then 2 or more equivalent DIs will be in the market.
Previous DI :
When UDI-DI is a replace of previous DI then old one shall be identified
If the same device has been imported to KSA market with different DIs, then 2 or more equivalent DIs will be in the market.
Previous DI :
When UDI-DI is a replace of previous DI then old one shall be identified
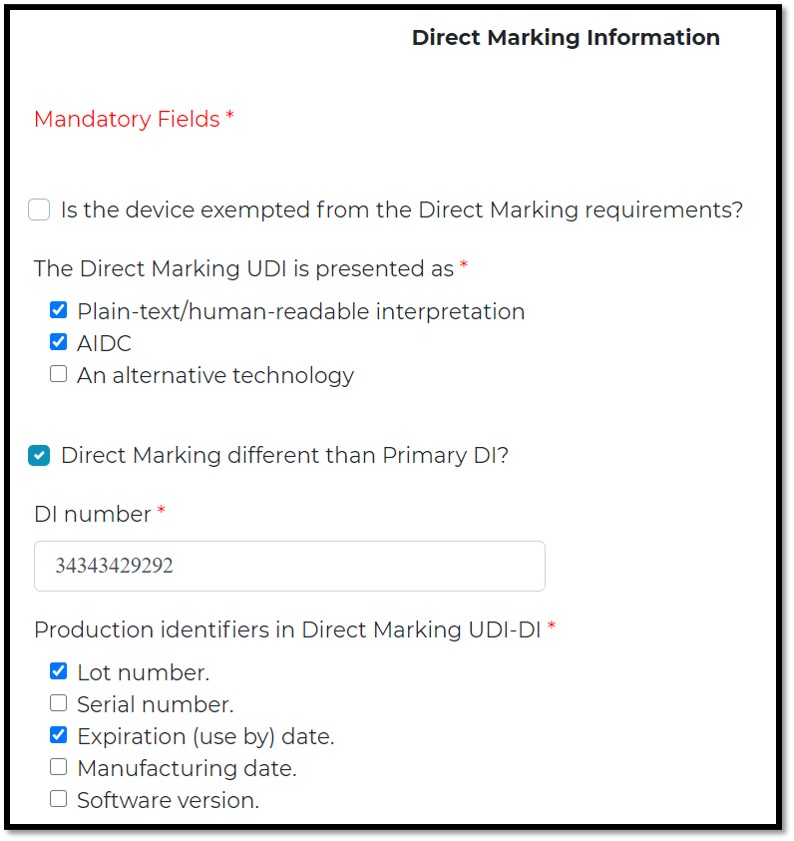
Direct Marking (DM) :
means a permanent marking providing the UDI on the device itself.
means a permanent marking providing the UDI on the device itself.

The DM UDI may be:
Identical to the UDI that appears on the label of the device, or A different UDI used to distinguish the unlabeled/unpackaged device. You can choose more than one identifiers
Identical to the UDI that appears on the label of the device, or A different UDI used to distinguish the unlabeled/unpackaged device. You can choose more than one identifiers
Devices packages levels:
start with information of the biggest package then the next smaller one, Until you reached the DI before the primary DI. the primary DI should not written here.

Devices packages levels:

Clinically relevant size:
as labeled, Device dimensions and parameters to select a specific device for a specific patient or procedures. (e.g. 7 cm , 10ml ,..) if there are multiple sizes as a kit then you can (add) them one by one.

MRI safety status : Is it compatible with MRI.
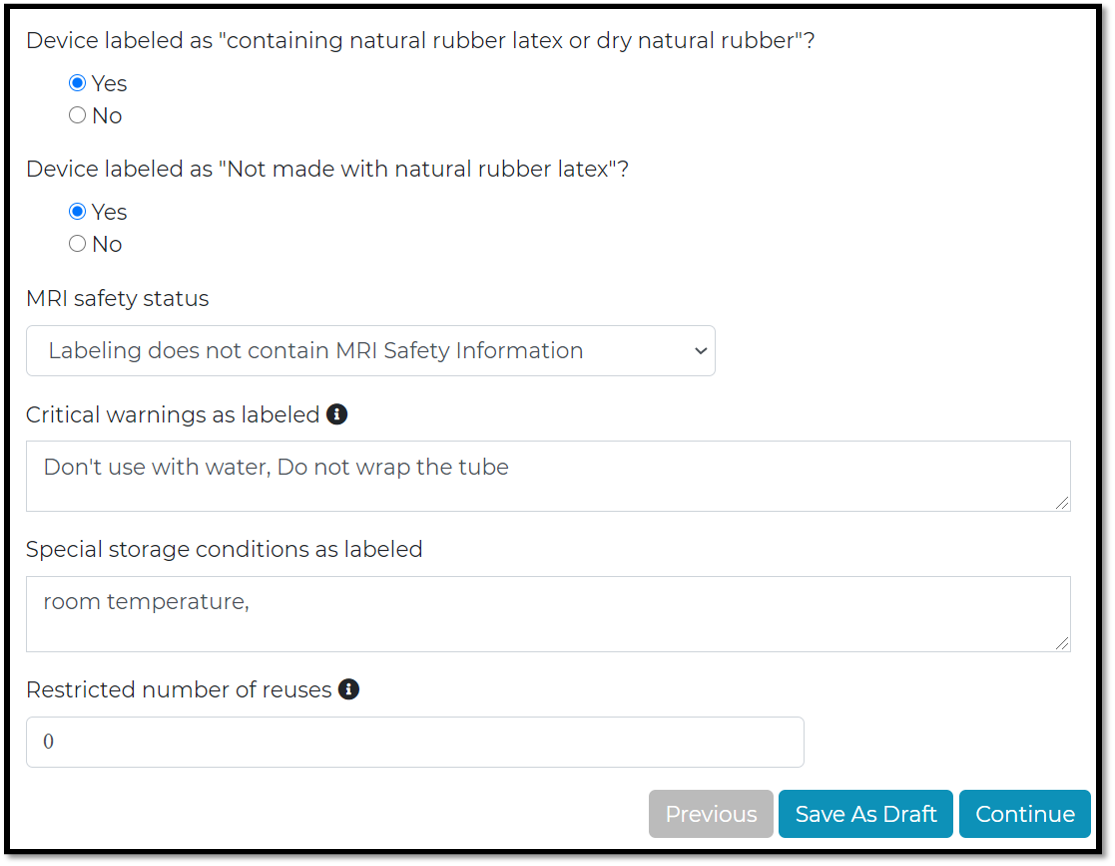
The last step, data will be recorded
Submit: will be registered and display in view page
Safe as draft: to check the information and complete any missing
Submit: will be registered and display in view page
Safe as draft: to check the information and complete any missing
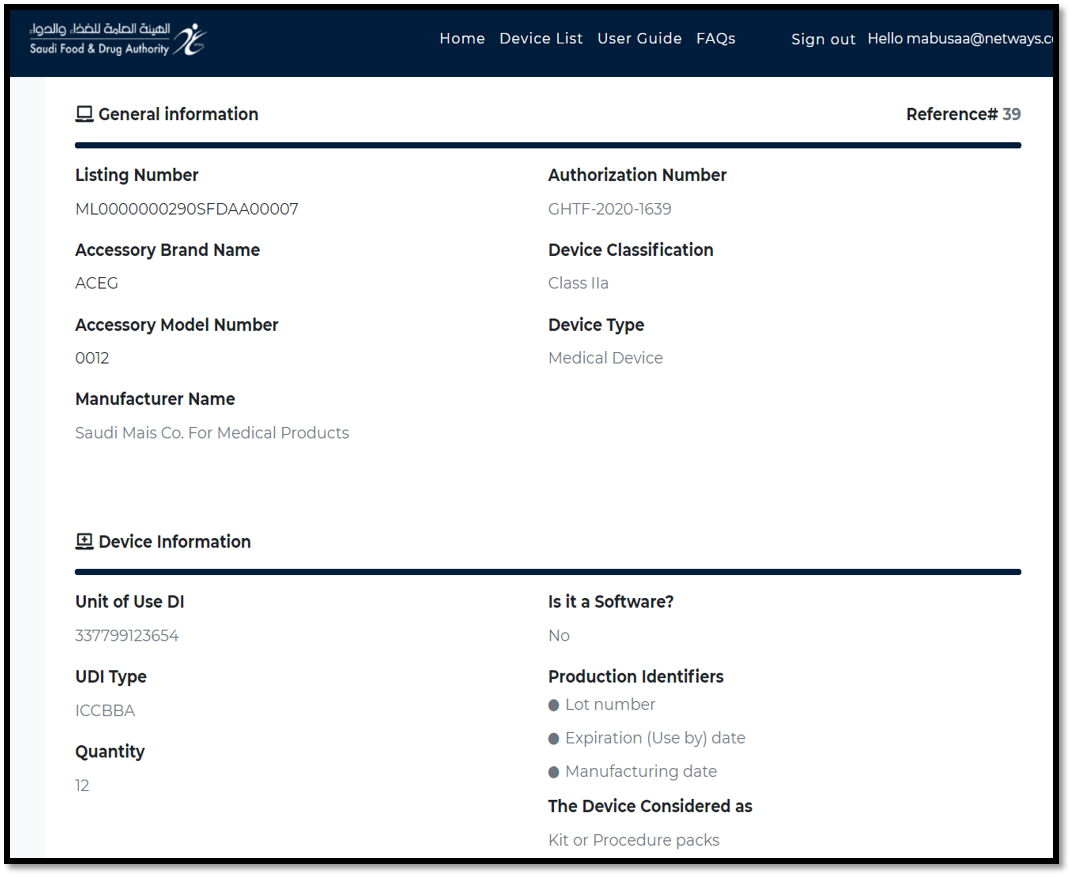
After submit, the View page will display all provided data of the registered device.

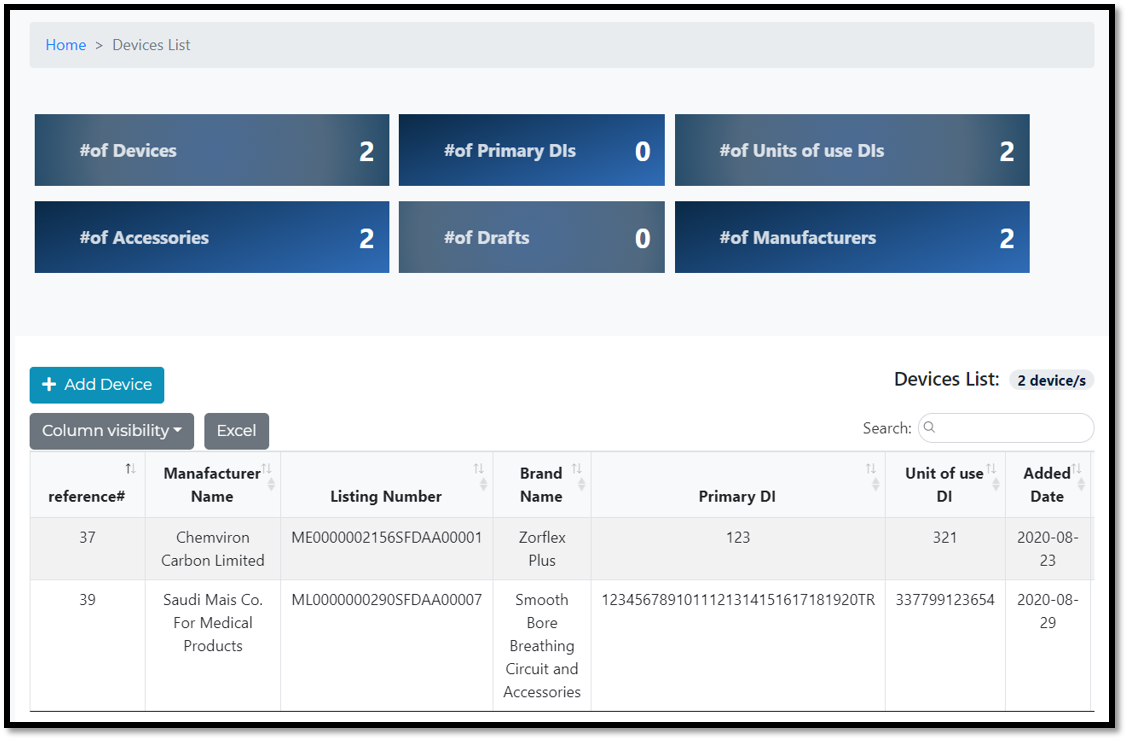
Devices list page :
Applicant can view all submitted data in one page
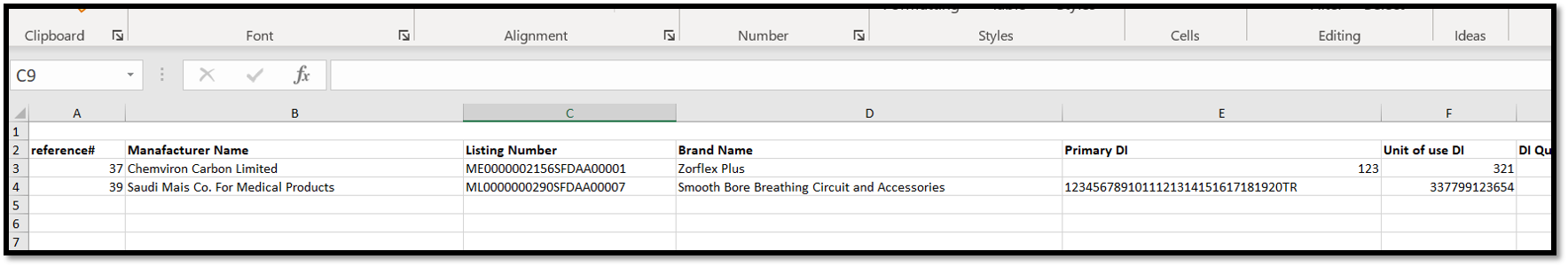
It also can be extracted in an excel sheet.
It also can be extracted in an excel sheet.